Our brain’s neurons are like a bustling city, where each building relies on a steady flow of electricity to function. In the event of a brief power outage, systems are in place to bring it back to life—no harm done.
But what if a power failure were to last for months? Emergency generators may keep essential services running, but eventually, they would fail. Water systems might freeze and burst, buildings would deteriorate, and infrastructures would start to crumble. When the power finally returned, the damage would be done—the city in ruins.
Lilianne Mujica-Parodi, lead author of a March study on brain aging patterns and interventions, shared the above analogy and said, “It’s easier to cure a problem while it’s still small.”
The study showed that aging follows a specific progression, with the first stage occurring in middle age and coinciding with higher insulin resistance.
Just like a city that suffers lasting damage when power is restored too late, the brain can reach a point where intervention is no longer effective. That’s why early action is crucial.
The Aging Brain
The brain follows distinct stages of decline—remaining stable until the mid-40s, when degenerative changes begin, and accelerating sharply by the mid-60s, according to Mujica-Parodi, director of the Laboratory for Computational Neurodiagnostics at Stony Brook University.
One key factor in brain aging is reduced glucose metabolism—the brain struggles to use carbohydrates for energy, impairing its function. These metabolic changes begin decades before symptoms appear but often go unnoticed until later stages of aging when intervention is far less effective. However, functional magnetic resonance imaging and electroencephalogram—tools used to study brain activity—can detect early age-related brain changes, providing an opportunity for prevention rather than late-stage treatment.
Understanding disease mechanisms is the first step toward effective treatment, according to Mujica-Parodi. For example, Alzheimer’s disease has long been attributed to the buildup of beta-amyloid, a protein that forms sticky plaques between brain cells, and tau proteins that form twisted tangles inside brain cells, leading to drug development aimed at clearing these proteins. However, these treatments have largely failed.
One reason for this failure is that by the time that Alzheimer’s disease is diagnosed, irreversible neuronal damage has already occurred. Protein accumulation is a consequence of insulin resistance in the brain—in other words, by targeting the beta-amyloid and tau proteins, the drug is failing to address the root cause.
Unlike many other cells, adult neurons have very limited regenerative capacity. If cognitive decline stems from neurons effectively starving, as the study suggests, waiting until they are incapacitated or dead is unlikely to be effective, according to Mujica-Parodi.
Physiological systems are designed to maintain homeostasis—a balance between energy supply and demand. When that balance is disrupted, the resulting stress can drive further dysregulation, worsening the problem over time, she said.
Once disruptions accumulate and secondary effects such as metabolic stress and glucose dysregulation take hold, fixing the original issue is no longer enough.
Insulin Resistance as a Major Driver
The first significant shift in brain network instability occurs alongside rising insulin resistance, often measured by HbA1c, a marker of long-term blood sugar levels.
Neurons rely on two primary energy sources: glucose and ketones. While some neurons require insulin to access glucose, those that become resistant to insulin struggle to utilize this fuel, a condition known as “insulin resistance,” Mujica-Parodi said.
As the cells lose their ability to effectively use glucose—their primary energy source—metabolic stress increases, slowing how messages are sent between nerve cells and contributing to cognitive decline.
In conditions such as Alzheimer’s disease, glucose uptake and utilization are impaired. This is why Alzheimer’s is sometimes referred to as Type 3 diabetes, Angel Planells, a Seattle-based registered dietitian nutritionist, told The Epoch Times.
When neurons become insulin-resistant, they lose their ability to access glucose but can still utilize ketones—which don’t require insulin for metabolism and provide an alternative energy source, Mujica-Parodi said.
Even in older adults with mild cognitive impairment or Alzheimer’s, it has been shown that brain cells can still take in ketones, although, by this stage, irreversible damage may limit their effectiveness.
That’s why identifying windows of intervention is crucial for proactively protecting the brain.
Windows of Intervention
“Cognitive decline tied to aging isn’t an inevitable consequence of growing older, but rather a process that may be prevented through early interventions targeting insulin resistance in the brain,” Mujica-Parodi said.
Brain aging follows a predictable trajectory. Unlike a gradual, linear decline, these changes occur in an “S-shaped” curve, suggesting specific windows during which interventions could be most effective.
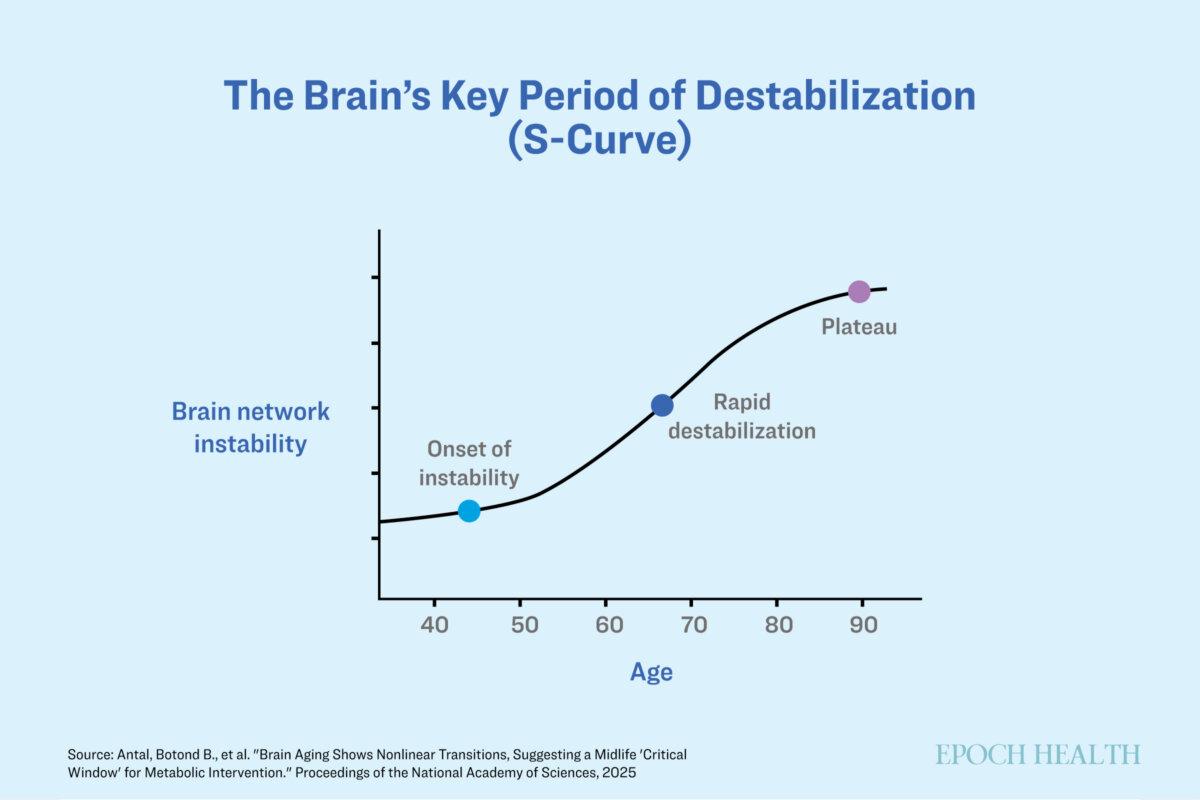
The average brain becomes more unstable over time, but metabolic interventions may help. (The Epoch Times)
From the late 40s onward, brain networks undergo significant changes marked by instability and loss of coordination. These changes are similar to those seen in people with Type 2 diabetes—reinforcing the idea that insulin resistance is a major factor in early cognitive decline.
The period between age 40 and age 60 is the most critical window for intervention. During this time, brain networks are most unstable but still adaptable, making it an optimal period for interventions.
A Keto Diet
Metabolic interventions that quickly bypass insulin resistance have proven to be effective, such as ketone supplementation or following a ketogenic diet.
Mujica-Parodi was surprised by how quickly these interventions took effect—in her studies, brain networks stabilized within just 30 minutes of consuming a ketone drink.
In one study, participants were given either a ketone or a glucose drink after an overnight fast, and their brain activity was measured using functional magnetic resonance imaging scans to observe any changes. They found that how well the brain’s networks are functioning changes with different fuel sources: glucose lowers stability, while ketones increase it. This effect was seen with both diet changes and ketone supplements, showing that the brain switches networks to save energy when resources are limited. A previous study also showed similar changes after just one week on a ketogenic diet.
Ketones can be produced in the body through low-carbohydrate, high-fat diets or fasting—or can be taken as a supplement—but brain health doesn’t need to wait until your 40s. Early lifestyle changes, such as adopting lower-carbohydrate, higher-fiber diets and engaging in regular exercise, can help prevent or delay insulin resistance in the brain, Mujica-Parodi said.
Once you hit your 40s, screenings for brain insulin resistance—beyond the standard HbA1c measures—could help identify risk early enough to implement ketogenic diets or supplements to support glucose access.
“Not everyone needs a strict keto diet,” Planells said. “But reducing processed carbs and improving insulin sensitivity generally benefits brain health.”
It should be noted that despite its potential benefits, ketone supplementation and the ketogenic diet come with limitations. For some, the restrictive nature of the ketogenic diet may reduce compliance, while ketone supplementation can cause side effects such as gastrointestinal distress, headaches, or electrolyte imbalances.
In addition to ketogenic diets and supplements, cognitive resilience—the brain’s ability to adapt to stress and maintain its function—can also be built through activities such as mentally stimulating tasks, learning new skills, and maintaining social connections, Planells said. Chronic stress and elevated cortisol levels can speed up brain aging—which is why mindfulness practices, such as meditation, are beneficial, he said.
“The window of opportunity may be narrow, but knowing it exists gives us the power to act,” he said.