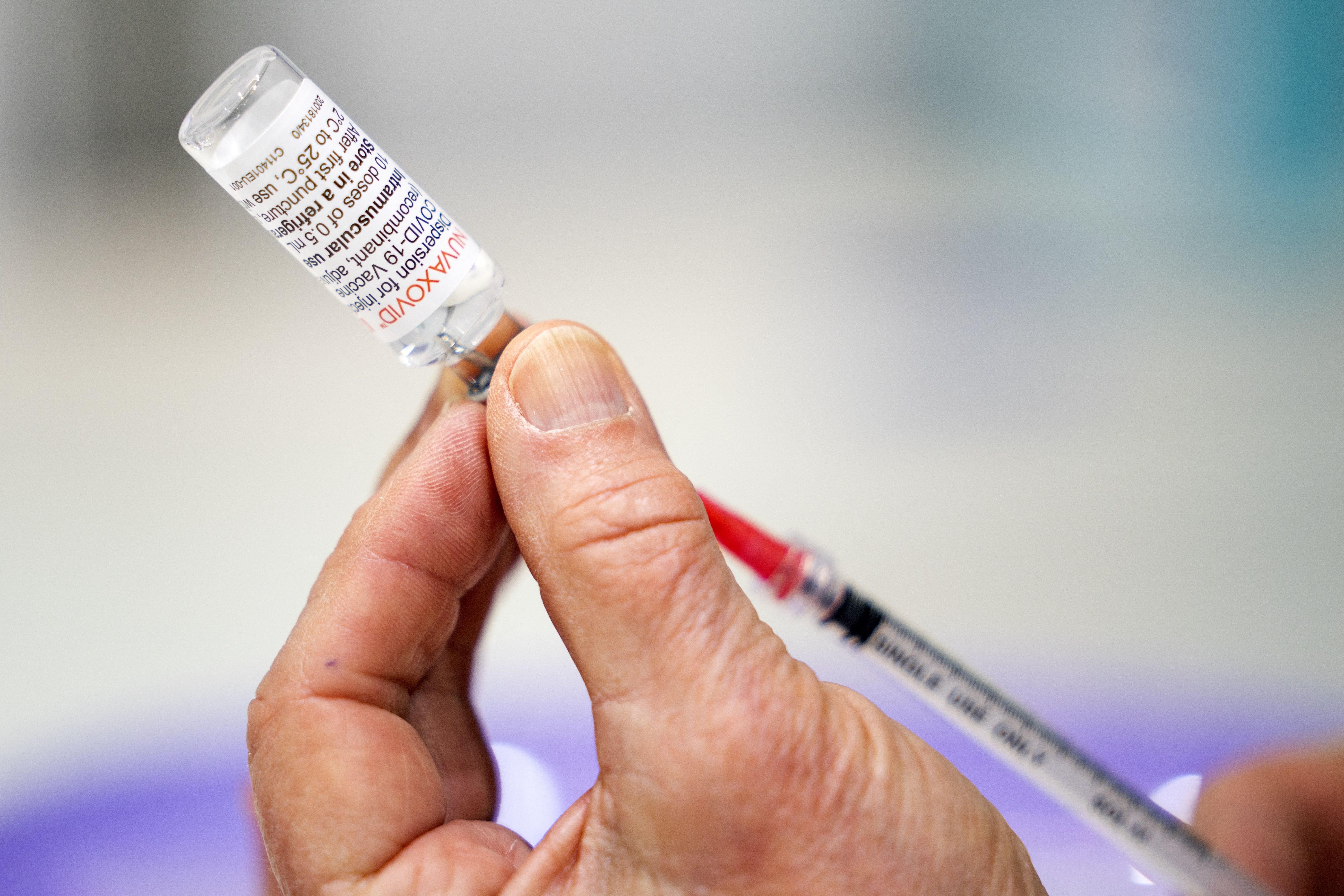
A company that makes one of the three COVID-19 vaccines available in the United States said on April 23 that its shot is on track for regulatory approval, after regulators declined to grant approval before a deadline.
“We believe that our Biologics License Application (BLA) is approvable based on conversations with the U.S. Food and Drug Administration (FDA),” Novavax said in a statement.
“We have recently received formal communication from the FDA in the form of an information request for a postmarketing commitment (PMC) to generate additional clinical data. We look forward to engaging with the FDA expeditiously to address the PMC request and move to approval as soon as possible.”
The FDA was supposed to decide on Novavax’s application for a BLA by April 2, but missed the deadline, the company has said.
“Any delays to the FDA’s independent review process for the Novavax are a result of scientific review to ensure safety and efficacy,” an official with the Department of Health and Human Services, the FDA’s parent agency, told The Epoch Times on April 3.
Health Secretary Robert F. Kennedy Jr. previously said that Novavax’s vaccine is not effective.
The FDA did not respond to a request for comment on Wednesday.
Dr. Tracy Hoeg, an assistant to the FDA’s commissioner, told a meeting on April 15 that the administration will provide an update on the Novavax vaccine soon.
The Centers for Disease Control and Prevention currently recommends that all individuals aged 6 months and older receive a COVID-19 vaccine, although the agency’s advisers recently said they’re considering advising the agency to narrow the recommendations.
Just 23 percent of adults and 13 percent of children have received a COVID-19 vaccine in the 2024–2025 season, which started in the fall, according to CDC data.
The FDA in 2024 granted emergency authorization to an updated version of shots from Novavax, Moderna, and Pfizer. Licensure requires a higher level of safety and effectiveness.
The FDA has approved the Moderna and Pfizer vaccines for most Americans, although they are still under emergency authorization for some children.
Novavax makes a protein-based shot, providing an alternative to the messenger ribonucleic acid platforms that Moderna and Pfizer use.
Novavax told investors in February it was expecting a BLA from U.S. regulators in April. If the company receives approval, Novavax will receive a $175 million payment from Sanofi under a partnership the companies reached to co-develop vaccines.
Novavax is also working with Sanofi on a combination COVID-19-influenza vaccine. That shot is being tested in phase 3 clinical trials.