The two largest pharmacy chains in the United States are no longer offering COVID-19 vaccines in some states, after federal regulators narrowed clearance for the shots.
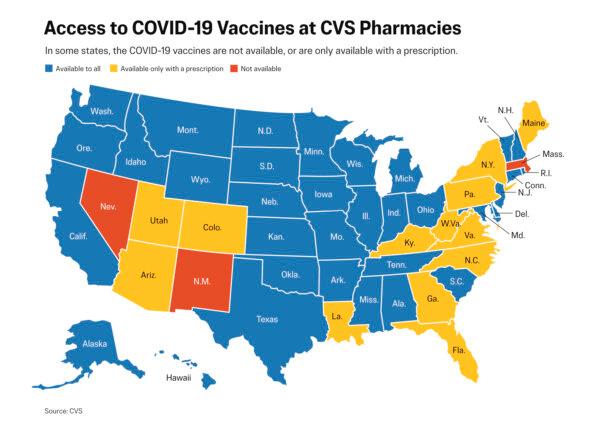
CVS is not offering the vaccines in three states—Massachusetts, Nevada, and New Mexico—a spokeswoman told The Epoch Times in an email on Aug. 29.
CVS pharmacies will only administer the vaccine to people with prescriptions in another 13 states, the spokeswoman said.
The Food and Drug Administration this week rescinded emergency authorization for the COVID-19 vaccines while issuing updated approvals.
The Centers for Disease Control and Prevention’s vaccine advisory committee, known as ACIP, is scheduled to discuss in a mid-September meeting to which populations the CDC should recommend the vaccines.
Laws in the 13 states and the District of Columbia prohibit administering a vaccine that does not have approval from the committee to patients without a prescription, the CVS spokeswoman said. Those states are Arizona, Colorado, Florida, Georgia, Kentucky, Louisiana, Maine, North Carolina, New York, Pennsylvania, Utah, Virginia, and West Virginia.
Laws in Massachusetts, Nevada, and New Mexico, meanwhile, “do not allow us to vaccinate without ACIP-approval, even with a prescription,” she stated.
The states where CVS is offering the vaccines without a prescription are Alaska, Alabama, Arizona, California, Connecticut, Delaware, Hawaii, Iowa, Idaho, Illinois, Indiana, Kansas, Maryland, Michigan, Minnesota, Missouri, Mississippi, Montana, Nebraska, North Dakota, New Hampshire, New Jersey, Ohio, Oklahoma, Oregon, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Vermont, Washington state, Wisconsin, and Wyoming.
(Illustration by The Epoch Times, Getty Images)
Dr. Georges C. Benjamin, executive director of the American Public Health Association, told The Epoch Times that the company’s position on state laws is correct.
Because the narrowed approvals came after the CDC stopped recommending the vaccines for healthy children and pregnant women, and happened just before the virus season started, “there is a great deal of uncertainty as to what the actual recommendation will be,” he said.
The FDA’s updated approvals are for the elderly and people under 65 who have obesity or one of the other conditions that the CDC says put them at “higher risk for severe COVID-19.”
The FDA on Aug. 22, 2024, cleared updated vaccines against COVID-19 for all individuals aged at least 6 months. The CDC’s advisers had advised the CDC ahead of time to recommend the vaccines for that population, and the CDC did so.
Walgreens
Walgreens, which did not respond to requests for comment, is not offering COVID-19 vaccines in eight states, according to attempts to schedule appointments through its website on Friday. Those states are Florida, Kentucky, Maine, Massachusetts, Nevada, North Carolina, North Dakota, and Pennsylvania.
In another 16 states—Arizona, Colorado, Georgia, Indiana, Louisiana, Missouri, Montana, New Mexico, New York, Oregon, South Carolina, Utah, Virginia, Washington state, West Virginia, and Wisconsin—Walgreens will only administer COVID-19 vaccines to people who have prescriptions, according to the portal.
The website also states that Walgreens will only provide COVID-19 vaccines to individuals who are at least 65 years of age, or younger people who have one of the risk conditions as defined by the CDC.
Moderna’s vaccine Spikevax is approved for individuals as young as 6 months of age.
The Pfizer-BioNTech vaccine is approved for people 5 years of age and older.
The Novavax vaccine and Moderna’s other vaccine are approved for people who are at least 12 years of age.
Moderna and Pfizer said that upon approval, they would be shipping doses within days.
A spokesperson for Sanofi, which is working with Novavax, told The Epoch Times in an email that Novavax’s vaccine will be available in the early fall.
“We expect to receive the updated 2025–2026 COVID-19 vaccines in the coming days,” the CVS spokeswoman said. “We’ll administer FDA-authorized COVID-19 vaccines in states where legally permitted at CVS Pharmacy and/or MinuteClinic to meet our patients’ needs.”
Will Insurers Cover?
Some health groups expressed concern that the narrower approvals would result in some people having to pay out of pocket for the vaccines, if they are able to find them at pharmacies or doctors’ offices.
Insurers are required to cover vaccines recommended by ACIP, under federal law. Insurers can also take into account recommendations from outside groups, such as the American Academy of Pediatrics, whose recommendations have diverged from the CDC’s updated schedules.
“We’re working closely with our members to review yesterday’s FDA announcement and will be monitoring the forthcoming meetings and recommendations from ACIP and CDC on considerations around coverage,” Tina Stow, a spokesperson for AHIP, an insurance trade group, told The Epoch Times in an email.
“Individual health plans and plan sponsors will be prepared to make coverage decisions informed by science, the latest medical evidence and data. This process will be evidence-based, evaluate multiple sources of data, including but not limited to ACIP, and will be informed by customer needs.”
Aetna, CVS Health’s insurer, is continuing to provide coverage for approved vaccines, such as the COVID-19 shots, “in compliance with applicable state and federal cost-sharing requirements,” a spokesperson for Aetna told The Epoch Times in an email.
“All members of insured plans voluntarily choosing to vaccinate against COVID-19 may do so with no cost sharing.”
Other major insurers, including UnitedHealth and Cigna, did not respond to requests for comment by publication time.