Experts share different views on the U.S. Food and Drug Administration’s (FDA) recent decision to expand its approval of FluMist, allowing the influenza nasal spray to be self-administered for the first time.
Pharmacists can prescribe the nasal vaccine and the spray can also be administered by a caretaker, the FDA said in a statement on Friday.
“For those interested in self- or caregiver-administration, the vaccine manufacturer plans to make the vaccine available through a third-party online pharmacy,” said the FDA.
These people would be screened to see if they are eligible. If they are, a third-party pharmacy will write the prescription and ship the vaccine to the address provided by the individual. People can also find a health care provider to administer it to them.
Dr. William Schaffner, a professor of preventive medicine at Vanderbilt University Medical Center, told The Epoch Times that the new approval may encourage vaccination, particularly in people who are afraid of needles or intramuscular inoculations.
“Many younger people who are used to dealing with the internet and having all kinds of packages sent to them may find this attractive,” he added, since not all doctors would be ordering the nasal spray vaccine.
Pediatrician Dr. Renata Moon disagreed with the FDA’s approval.
The FDA states that the pharmacy can write the prescription for the vaccine. “Pharmacies are now allowed to practice medicine?” Dr. Moon said in an email to The Epoch Times.
She argued that this new decision could circumvent informed consent discussions between physicians and patients.
When the product was introduced, there were concerns from clinicians that there may be risks if a person receives it from a non-health professional, Dr. Schaffner said.
However, AstraZeneca, the manufacturer of the spray, conducted a study in 2015 showing that 100 percent of adults could appropriately self-administer the full dose when given instructions.
It is currently unknown if self-administration would present any higher risks than being given the vaccine from a health practitioner, Dr. Schaffner added.
The spray will be available for consumers in fall 2025, the company’s spokeswoman told The Epoch Times.
Precautions
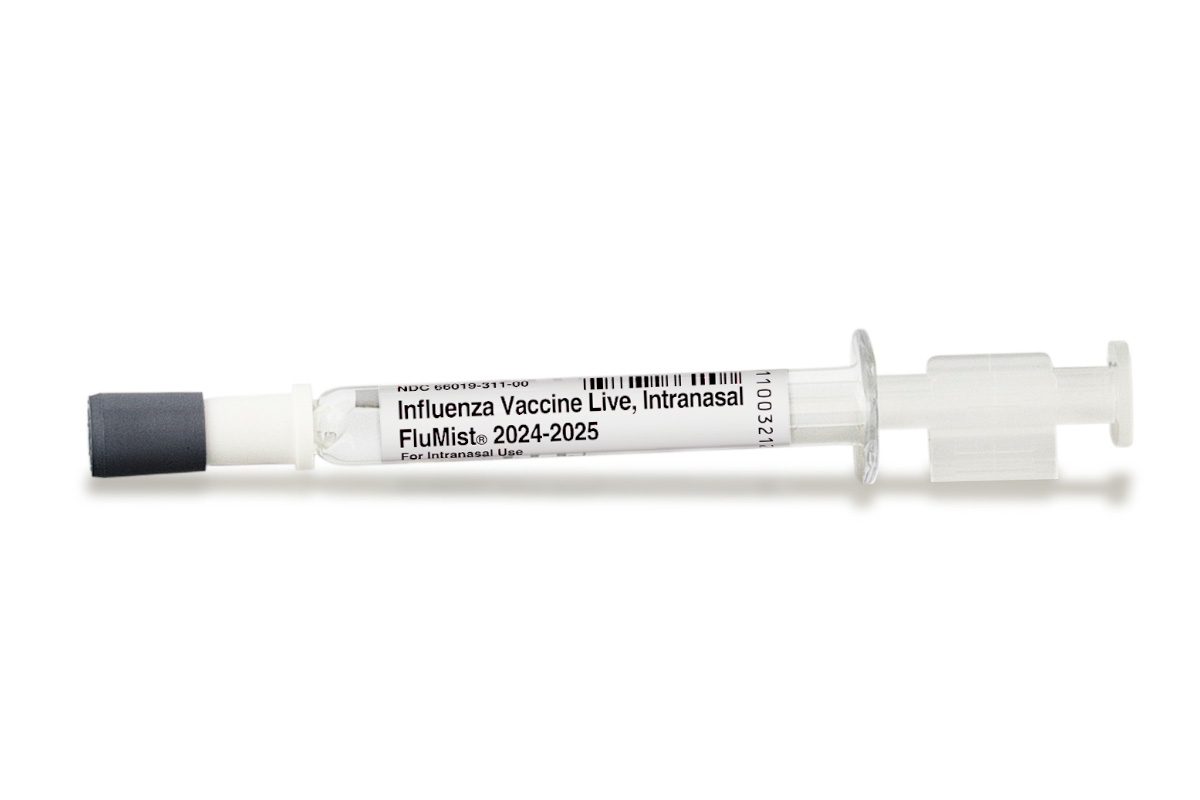
FluMist is currently the only FDA-approved nasal vaccine. The FDA approved it in 2003 for use in individuals aged 5 through to 49, and broadened the age range in 2007 to also include children aged 2.
Contrary to most intramuscular influenza vaccinations, FluMist contains live weakened viruses rather than killed influenza viruses.
People who are pregnant, immunocompromised, or living with people who are immunocompromised are therefore at risk of potential infection and should check with their health provider if they want to receive this vaccine, according to the vaccine’s prescription label.
People who have had a history of wheezing, Guillain-Barre syndrome, or have problems with heart, kidney, lungs, or diabetes should also confirm with their family doctor.
The nasal vaccine is administered by spraying half a dose to each nostril separately.
According to the drug’s prescription label, people should not use FluMist if they have an allergy to eggs, are taking aspirin or medicines containing aspirin, or are allergic to other inactive ingredients in the vaccine.
The most common side effects are a runny or stuffy nose, sore throat, and running a fever.