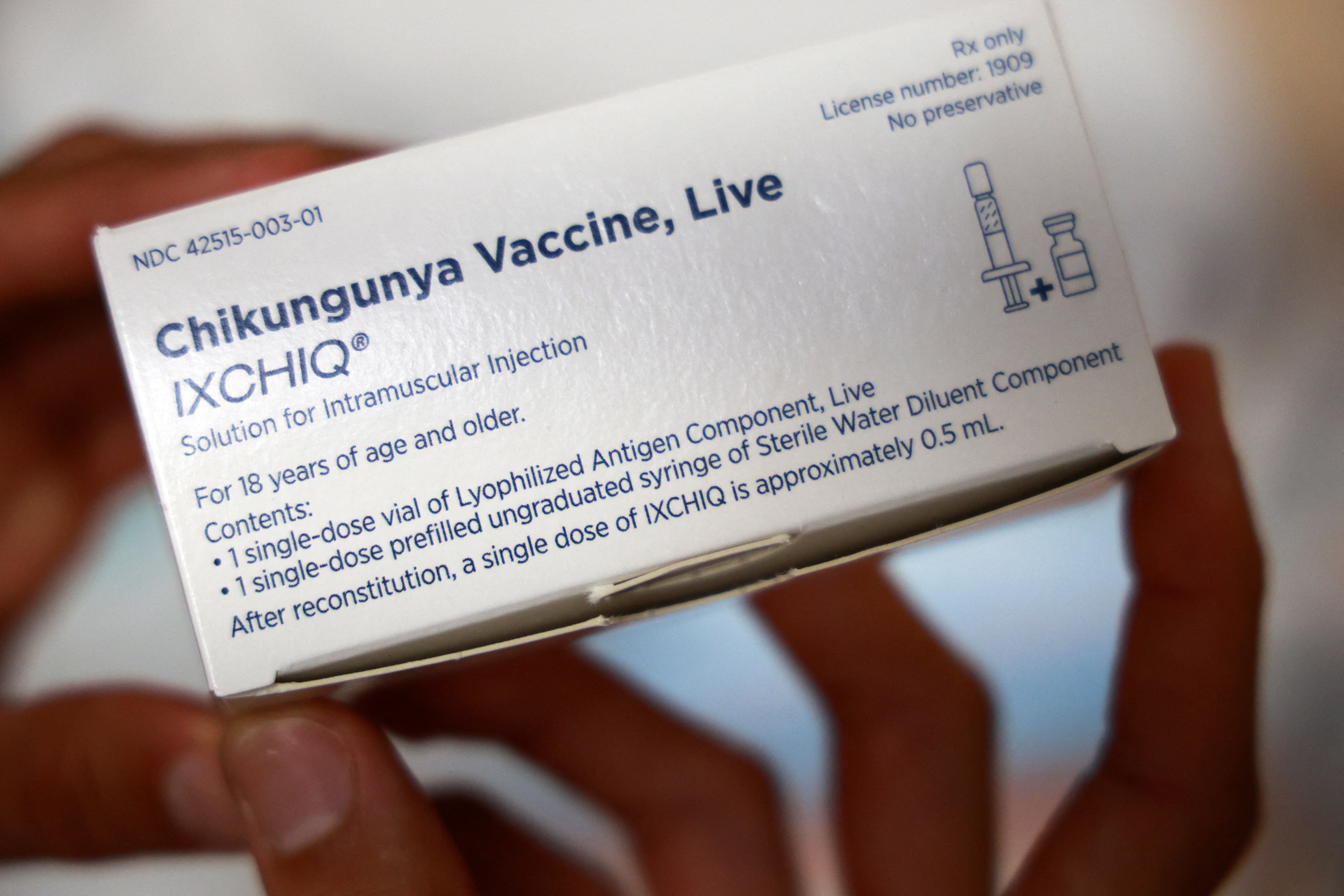
Federal officials paused administration of a vaccine against the chikungunya virus for some adults because post-vaccination problems may be related to the shot, regulatory authorities said on June 5.
Seventeen serious adverse events—including two deaths—had been reported by early May among people who received the chikungunya vaccine.
“FDA’s pause is intended to allow the agency time to adjudicate these events and ascertain whether additional, unreported adverse events exist,” Dr. Vinay Prasad, the Food and Drug Administration’s top vaccine official, and two other FDA officials said in an article published by the Journal of the American Medical Association.
Definitively attributing the events to vaccination requires looking at various factors, including whether vaccine recipients were also infected with chikungunya, the officials said.
The FDA approved the vaccine, made by Valneva and known as Ixchiq, in 2023. The Centers for Disease Control and Prevention in 2024 recommended it for adults traveling to some foreign countries.
The vaccine contains a live, weakened version of the virus and has been known to cause symptoms similar to those caused by the virus, the FDA says in a label for the shot.
After learning about the adverse event reports, the FDA and CDC on May 9 advised doctors to stop administering the vaccine, or essentially imposed a pause, to adults aged 60 and older while the agencies investigated.
“FDA will conduct an updated benefit-risk assessment for the use of Ixchiq in individuals 60 years of age and older. In addition, FDA and CDC will continue the evaluation of postmarketing safety reports for Ixchiq,” the agencies said at the time.
The investigation is still underway, Prasad, Dr. David Kaslow, and Dr. Sixun Yang said in the new article.
“Although more information must be gathered, the potential that the observed serious adverse events may be related to the vaccine in people older than 60 years, in those with comorbidities, or both merits serious investigation,” they wrote.
Valneva has said it is working with authorities in the United States and Europe. The company said it sees a positive risk-benefit calculus in the vast majority of people who are potentially exposed to chikungunya.
The vaccine was given accelerated approval after it triggered an immune response to chikungunya among participants in two clinical trials. Two additional trials were required following approval, but neither has begun yet, “so FDA cannot provide any additional risk-benefit assessment” for older adults, the FDA officials said.
The FDA is still committed to accelerated approval of vaccines for rare diseases such as chikungunya, according to the article. The officials wrote that the agency “will continue to prioritize increasing availability of safe and effective products for rare, serious, and neglected diseases without available therapy and continue to refine its methods to ensure the rapid development of confirmatory evidence to better inform risk-benefit assessment considerations.”