Scientists have found a new antibiotic that targets the main culprit behind gum disease.
In an August study published in the Journal of Oral Microbiology, researchers found that a narrow-spectrum antibiotic, FP-100, eliminated bad bacteria called Fusobacterium nucleatum in mice—without harming beneficial bacteria—and “significantly” reduced bone loss that had resulted from the condition.
“This type of black-and-white data almost never happens,” Dr. Alpdogan Kantarci, a senior scientist at ADA Forsyth and lead author of the study, said in a statement.
If successful in people, this targeted approach can improve oral health and help prevent gum disease from spreading beyond the mouth. However, experts caution that human trials are still on the horizon.
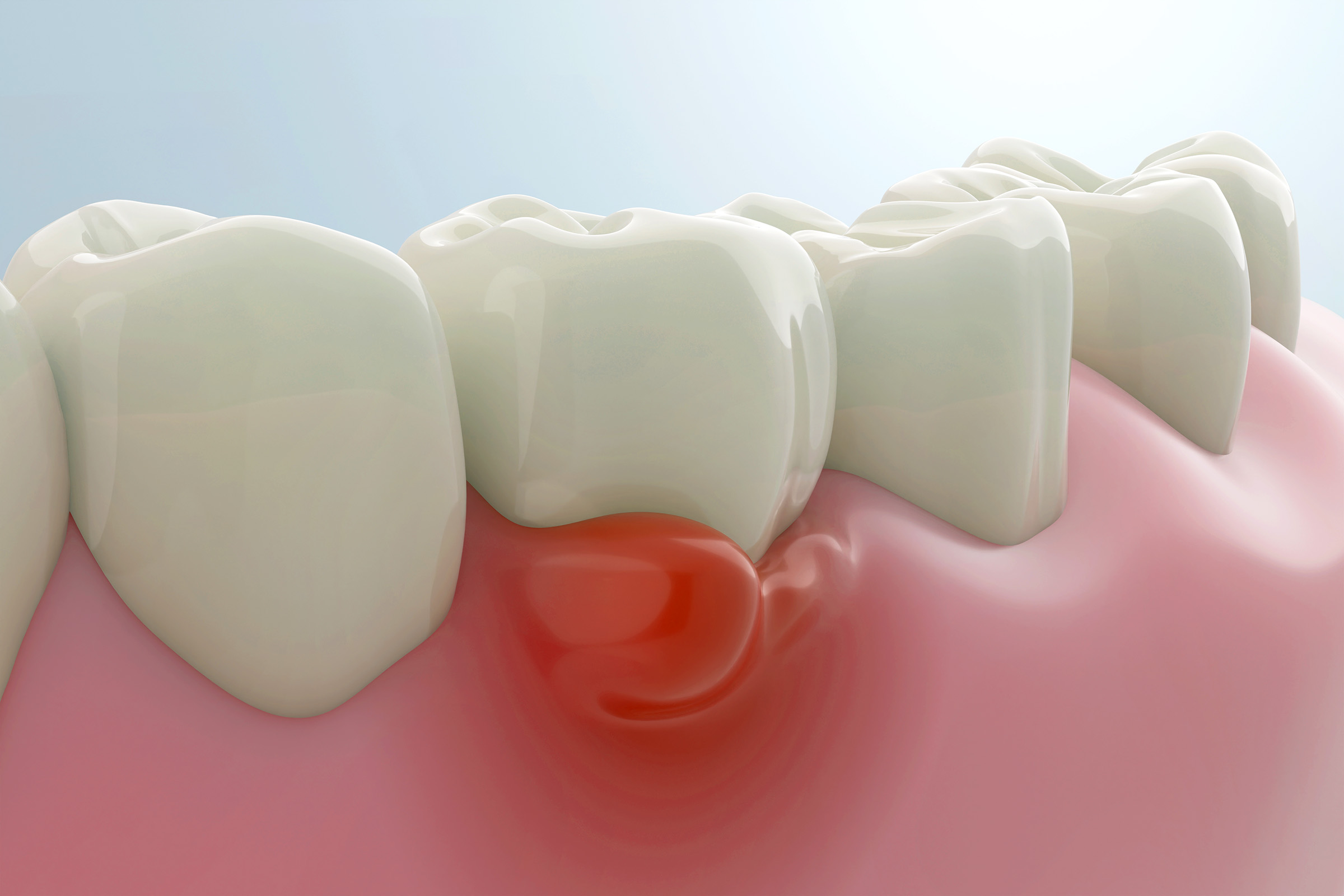
The Main Culprit
Several bacterial species have been found to initiate and cause the progression of gum disease, with F. nucleatum being one of the
most abundant in these diseases. F. nucleatum also
increases in number, along with other harmful bacteria, as gum inflammation worsens.
In the mouth, F. nucleatum bacteria form dental biofilms, which are a layer of slimy bacterial coating on the teeth. The microorganisms invade gum cells and weaken the immune system, which in turn promotes bacterial invasion and gum disease.
F. nucleatum can also hide inside immune cells, which act like Trojan horses to transport the bacteria to distant organs, according to Kantarci. As a result, some studies have suggested that bacteria may be linked to the development of chronic diseases, including heart disease, Alzheimer’s disease, and colorectal cancer.
A Targeted Approach
FP-100, the narrow-spectrum antibiotic, may be effective in treating periodontitis without harming beneficial bacteria, researchers say.
Periodontitis, a severe gum disease, develops when untreated inflammation causes bacteria to collect between gums and teeth, affecting tooth roots and surrounding bone, and eventually leading to tooth loss.
Gum disease isn’t curable but can be managed with good oral hygiene, deep cleaning of the roots, and—in severe cases—surgery. Dentists sometimes prescribe antibiotics to supplement mechanical treatments.
Broad-spectrum antibiotics such as amoxicillin are typically used for treatment supplementation, Dr. Pierluigi Balice, a board-certified periodontist at Newton Dental Associates in Massachusetts, told The Epoch Times.
However, broad-spectrum antibiotics kill both harmful and beneficial bacteria, Dr. Jason Cellars, a dentist at Seacliff Dental, told The Epoch Times.
“Basically, antibiotics are risk factors for opportunistic pathogen infections,” Sean Gibbons, associate professor at the Institute for Systems Biology, told The Epoch Times.
In the study, researchers tested FP-100 on cultured bacteria and mice with induced gum disease.
FP-100 significantly reduced F. nucleatum in the bacterial culture. It could kill F. nucleatum at low doses without significantly affecting the other microbes in the mouth. High doses of FP-100 caused a significant change to the overall microbe composition on day two.
Mice treated with FP-100 had no detectable F. nucleatum compared with the control mice, in which every sample had detectable F. nucleatum colonies. The treated mice also showed less bone loss and inflammation than the control group, suggesting that targeting F. nucleatum specifically can effectively reduce gum disease.
Promising, But Still Too Early
The researchers emphasized that the targeted elimination of F. nucleatum could break the link between oral and systemic diseases, potentially reducing risks of conditions such as colorectal cancer.
However, experts urge caution.
While the study’s concept is grounded in solid scientific reasoning, it is important to note that the research is currently based on an animal model, representing a low level of scientific evidence, Balice said.
“Similar approaches targeting specific bacteria have been explored in several microbiology studies in the past without resulting in clinical applications,” he said. “While I am hopeful that this study can be translated to human applications, I remain cautious.”
“There is no single animal model that mimics all the characteristics of human periodontitis and its tissue architecture or healing,” according to a 2024 study on periodontitis treatment.
Balice said that targeted antimicrobials are not frequently used in clinical practice because of limited evidence when tested in human randomized clinical trials.
Cellars said that the current findings do not justify the antibiotic’s use in periodontitis.
The main issue with periodontitis is that it is a slew of negative bacteria and physical plaque that causes the inflammation and disease, he said. Eliminating one strain of harmful bacteria doesn’t solve the problem because other bacteria will simply take its place, continuing the disease process, he noted.
“[Periodontitis] is a chronic long-term disease, which makes it difficult to study because results of treatment need to be tracked over years and not over months or weeks,” Cellars said.
While the study has shown that FP-100 does not affect the native microbiome, “most narrow spectrum antibiotics do target a subset of commensals,” Gibbons said, adding that super-narrow antimicrobials tend to be harder to find and develop.
Other emerging treatments for periodontitis include plant extracts, probiotics, and drugs that inhibit the immune system.