Levels of Acidosis
Acid-base homeostasis occurs when the body functions properly. At this stage, our systems can regulate the pH of our body’s extracellular fluids and are not negatively influenced by small amounts of excessive acid.
Latent Acidosis
Also called hidden acidosis, this form is characterized by elevated acidity levels in the body’s systems. However, the blood will most likely not show elevated pH levels yet, as the kidneys are still compensating and excreting acid through the urine.
Acute Acidosis
Acute acidosis is reached when the blood and other buffer systems are thrown over the threshold and can no longer compensate for high acidity levels in the body. This means that the acid-base balance is disturbed, but only for a limited period of time.
Chronic Acidosis
Chronic acidosis means that the condition has continued for an extended period. In this case, patients often hear that the cause of their illness is unknown. This can include rheumatism or other serious disease patterns.
Signs of Hyperacidity
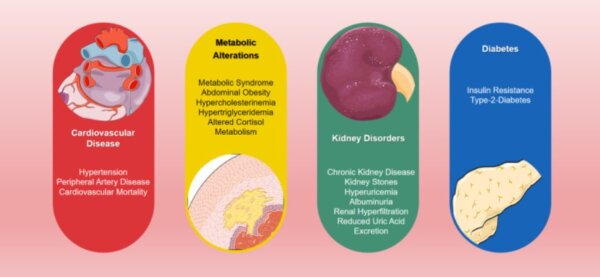
In hyperacidity, the body tries to rid itself of excessive acids, which become toxic at this point. This can also lead to high levels of phosphate in the blood called hyperphosphatemia, which can cause symptoms such as brittle nails and thin hair, bad breath, excessive perspiration, a wan skin tone, or a white or brown coating of the tongue. Hyperphosphatemia is caused by metabolic and respiratory acidosis.
Testing Your Body’s pH Levels
A primary care physician can test your blood acid levels. The serum uric acid blood test can be very insightful. Uric acid is produced when the body breaks down food. The test can show how well the kidneys filter this acid from the body, hence determining the body’s kidney function, which reveals the body’s capacity to buffer for dietary acid loads. Specific sensitive testing can be performed via the Liquid Chromatography-Tandem Mass Spectrometry Method, which can determine the levels of intracellular and extracellular uric acid.
Tracking Your Urinary pH
Urine tests should be performed five times per day for multiple days. Keeping a food diary at the same time is helpful to link possible dietary culprits to a possibly raised pH level. Therefore, do not change your eating habits during the test period. Also, refrain from taking any supplements or detoxification products for two days before and during testing.
Urine Test Schedule
It is important to eat only three meals on the days of testing, sticking with the following recommended timeframe. Ensure that you take a mid-stream-urine sample.
6 a.m.—Usually, the first urine in the morning will be the most acidic. At night, the liver tries to rid itself of accumulated acids. Also, the lack of food intake leads to missing alkaline input overnight.
9 a.m.—Perform this test 2 to 3 hours after breakfast, which you should have right after the first testing. At this time, the test will most likely be influenced by a flood of alkaline breakfast material. Therefore, in healthy people, this test’s results often show up as slightly alkaline.
Noon—This test should be conducted just before eating lunch. Now, the alkaline influence from breakfast has subsided.
3 p.m.—Normally, this test marks a deep dive into the alkaline zone because of the food eaten at lunch.
6 p.m.—This test is taken just before dinner. Being without food for six hours will make the curve go up and potentially dip into the acidic area of the measured value.
For your convenience, these times can also be moved one hour forward if this better fits your schedule.
Using Baking Soda
Sodium bicarbonate, also known as baking soda, is frequently used as a home remedy for heartburn or acid reflux. Be careful, however, since the “anti-acid” was shown to potentially increase blood pressure and harm the respiratory system. In a case review published in the Journal of Medical Toxicology, authors noted that by interfering with acid-base levels, baking soda can also harm the heart and respiratory systems.
Dietary Choices Are Key
A 2024 systematic review and meta-analysis published in Nutrition, Metabolism, and Cardiovascular Disease highlights the risk factors of a highly acidic diet on the human cardiometabolic system.
Storz MA, Ronco AL, Hannibal L. Observational and clinical evidence that plant-based nutrition reduces dietary acid load. J Nutr Sci. 2022 Oct 31;11:e93. doi: 10.1017/jns.2022.93. PMID: 36405093; PMCID: PMC9641522.
Authors of an analysis of the 2022 U.S. National Health and Nutrition Examination Survey, published in Food Science & Nutrition, also emphasize the importance of acid-base homeostasis for overall health.
The Alkaline Diet
According to the alkaline diet, 80 percent of your daily food intake should be alkaline food. The remaining 20 percent can be healthy acidic foods, meaning products that are only mildly acidic and are rich in other important minerals and nutrients, such as legumes, whole grain carbohydrates, certain types of nuts and vegetables (artichokes, asparagus, and Brussel sprouts), as well as green and white tea.

Most Acidic Foods and Drinks
Here is a list of the 10 most acidic foods:
- Soda
- Sugar
- Processed meat
- Alcohol
- Cheese (and certain other dairy products)
- White flour
- Seafood
- Fried food
- Heavily processed food
- High-protein foods and supplements
Most Alkaline-Increasing Foods
When trying to incorporate alkaline foods into your diet, the preparation also needs attention. Fruits are ideally harvested ripe and only minimally processed at home, if needed. Vegetables should be gently steamed or cooked.
- Bananas
- Bitter lettuce varieties, such as radicchio or chicory
- Broccoli
- Carrots
- Cauliflower
- Celery root & stalks
- Fennel
- Garlic
- Grapefruit
- Green and white beans
- Herbs (a great variety)
- Kohlrabi
- Mushrooms
- Onions
- Potatoes
- Pumpkin
- Red beets
- Spinach
- Tomatoes
- Lentils
Alkaline Recipe for the Season
Pumpkin Soup
Ingredients:
- 1 onion
- 1 potato
- 1 tbsp. extra-virgin olive oil
- 250 gms pumpkin of your favorite variety
- 1/2 liter broth
- Ginger, salt, pepper, curry
Instructions:
- Cut onion into small cubes and brown in pan with olive oil.
- Clean and cut other vegetables and cook in the broth for about 10 minutes or until soft. Puree with immersion blender.
- Add spices to taste.
Voices of Opposition
Many health professionals do not assign much value to an alkaline diet. First, they note a lack of research on the topic. Second, they claim that in a healthy person the lungs and kidneys continuously work to ensure a rightly regulated blood pH, no matter the dietary choices.
Alkalosis—Or too Much Alkalinity?
Alkalosis is contrary to acidosis. When the body’s pH level is more than 7.45, it is considered highly alkaline. This state can cause problems. Once again, the body employs its buffer mechanisms and the kidneys try to regulate.